Understanding the regulations and critical characteristics of personal protective equipment (PPE)
By Dr. Davis Lee; Dr. Erin Kirkpatrick; Dr. A. Sydney Gladman; Dr. Emily Fitzharris; Michael Posson, M.P.H., CIH; and Dr. Mark Roberts1
Textiles have long been used in protective coverings2, including gowns, coveralls, caps, and face coverings. In recent months, there has been much interest and activity from the healthcare industry, broader workforce, and general public regarding the use of protective coverings to reduce the risk of infection by the novel coronavirus (SARS-CoV-2). Consequently, there has been a sharp increase in demand for these products.
In addition to regular use of personal protective equipment (PPE) in healthcare and occupational settings, this increased demand results from the Atlanta-based Centers for Disease Control’s (CDC’s) recommendation that the public wear “simple cloth face coverings to slow the spread of the virus and help people who may have the virus and do not know it from transmitting it to others.”3 These face coverings are loosely defined as “simple cloth face coverings fashioned from household items or made at home from common materials at low cost.”3 By recommending the use of simple do-it-yourself (DIY) face coverings for the general public, has the COVID-19 pandemic created a unique opportunity for the textile industry to respond to the public need?
This article explores the regulations associated with PPE, surgical masks and unregulated protective face coverings. The specific regulatory definition of PPE, the performance standards and regulations that need to be met, and the pragmatic considerations related to fabric construction and end-use performance will be discussed. The information collected for this article is based on scientific and open sourced literature available at the time of this writing.

are made using nonwoven materials. Image: Mika Braumeister/
Unsplash.
Understanding The Regulatory Complexities Of PPE: Who Makes The Rules?
PPE has become a household term, but regulatory complexities and associated performance requirements are not commonly understood. In fact, PPE is a specific term regarding workplace protective equipment that is regulated and enforced by the Occupational Safety and Health Administration (OSHA) and other agencies. OSHA defines PPE as “equipment worn to minimize exposure to hazards that cause serious workplace injuries and illnesses.”4 Especially within the context of disease transmission, it is important to note that PPE is designed to protect the wearer, not necessarily others who may come into close proximity to the wearer.
Much of the discussion regarding protective coverings as it concerns the current COVID-19 pandemic is focused on protective face coverings. Along with OSHA, the United States Food and Drug Administration (FDA) and National Institute for Occupational Safety and Health (NIOSH) have been involved in providing guidance for protective coverings. While collaborative, the respective roles of these agencies are different. The scope of the FDA’s authority includes regulatory oversight of medical devices.5 NIOSH, on the other hand, is a research agency attached to the CDC “focused on the study of worker safety and health, and empowering employers and workers to create safe and healthy workplaces.”6 NIOSH also is under the jurisdiction of OSHA, and is the certifying agency for respiratory protection in workplaces in the United States.

respirator masks
Image: CDC/Pexels
What Are The Regulations?
PPE has very specific regulatory meaning. With regards to protective face coverings, there is a clear regulatory difference between PPE used in an occupational or healthcare setting — for example N95 respirators or surgical masks — and face coverings that may be used by the general public such as DIY face coverings as described by the CDC.3 For example:
- Properly fitted N95 respirators for industrial use are capable of filtering out at least 95 percent of 0.3 micron size particles.7,8,9,10 In the United States, PPE such as N95 respirators carry labels that designate the approval level — N95 — and approval by NIOSH under 42 CFR Part 84. NIOSH and the approved use — N95, for example — will be printed directly on the mask, and the packaging will be accompanied by information regarding OSHA compliance requirements and workplace hazards. In this case the employer is responsible for assuring that PPE is prescribed in accordance with the appropriate specifications, laws and regulations.
- Surgical masks for medical use are cleared by the FDA as a medical device but may or may not have NIOSH labeling or certification, depending on the product class and code.8,9,11 Surgical masks differ from respirators in their indications for use. Specifically, the respirators are intended to filter small particles and bio-aerosols, whereas surgical masks are typically designed to fit the face more loosely and serve to act as a barrier to the transfer of large droplets of saliva and/or mucus.12,13
- Face coverings for general public use as defined by the CDC do not currently have a regulatory mandate, and are not required to adhere to any governmental specifications or regulations.14 Likewise, protective face coverings colloquially referred to as “surgical masks” that are for general public use and intended for a non-medical purpose are not required to meet governmental specifications or regulations. Protective face coverings used in this context are not technically considered PPE.8,9,15
Regardless of the type of protective covering chosen, it should always be used in conjunction with appropriate and basic hygiene practices, such as properly donning and doffing coverings or PPE, and then washing one’s hands.16,17
What Are The Performance Standards?
If a respirator is to be used in a workplace as a PPE device under OSHA rule, it must go through a NIOSH certification process dictated in 42 CFR 84.18 There are also other performance requirements or industry standards associated with different types of PPE, for example ANSI/ISEA Z87.1,19 ANSI/ASSE Z88.2,20 and ASTM F2100.21 All of these are standards associated with the finished product. On the other hand, DIY face coverings, such as those recommended by the CDC, are not required to be tested under any government performance standards regarding filtration or personal protection.22 Thus, there is an opportunity for the textile industry to characterize typical functional properties and potentially develop minimum performance specifications based on current regulatory requirements of face coverings.
In addition to the product characteristics, a critical factor to consider with respirators and face covering performance is the fit to the user’s face. Airflow will follow the path of least resistance. If the covering does not tightly contour the face, air will leak through gaps between the face and seal, reducing filtration effectiveness. In this respect, there is a critical distinction between respirators — which are regulated under OSHA — and face coverings, which are recommended by the CDC for the general public. For example, N95 respirators used in medical or occupational settings are required to be fit-tested to ensure the individual fit and filtration efficiency for the user. Further, the user is required, as facilitated by the employer to undergo medical examination, training, and other requirements to assure that they are able to wear a respirator during the course of their employment. In contrast, since general protective face coverings may fit the user’s face more loosely, filtration performance of the textile material may be reduced.22

Use Of Textiles In Protective Coverings
It is generally understood that different protective coverings provide different levels of protection for the wearer and those around them. In these protective coverings, textile products are popular material choices because they simultaneously provide comfort and protection. For example, face masks and surgical masks used as PPE are typically constructed from nonwoven fabrics using fibers such as polypropylene, polyester and rayon.23 However, conventional knit or woven fabrics also are used for certain protective coverings. A variety of fiber and fabric options are available for DIY face coverings.
A review of the literature shows that many researchers have evaluated the utility and function of N95 respirators, surgical masks, and DIY face coverings. One study that evaluated the filtering efficiency of DIY face coverings and surgical masks for particles less than 10 microns found that the filtering efficiency of DIY face coverings ranged from approximately 60 to 80 percent, depending on the fabric characteristics, as compared to greater than 90 percent efficiency for the surgical masks, indicating that face coverings provide some level of protection for the general population.24 Studies have found that face coverings “reduced emitted particles (leakage) by 1/5, surgical masks reduced it by 1/2, and N95-equivalent masks reduced it by 2/3” for particles 0.02-1.0 microns in size.25
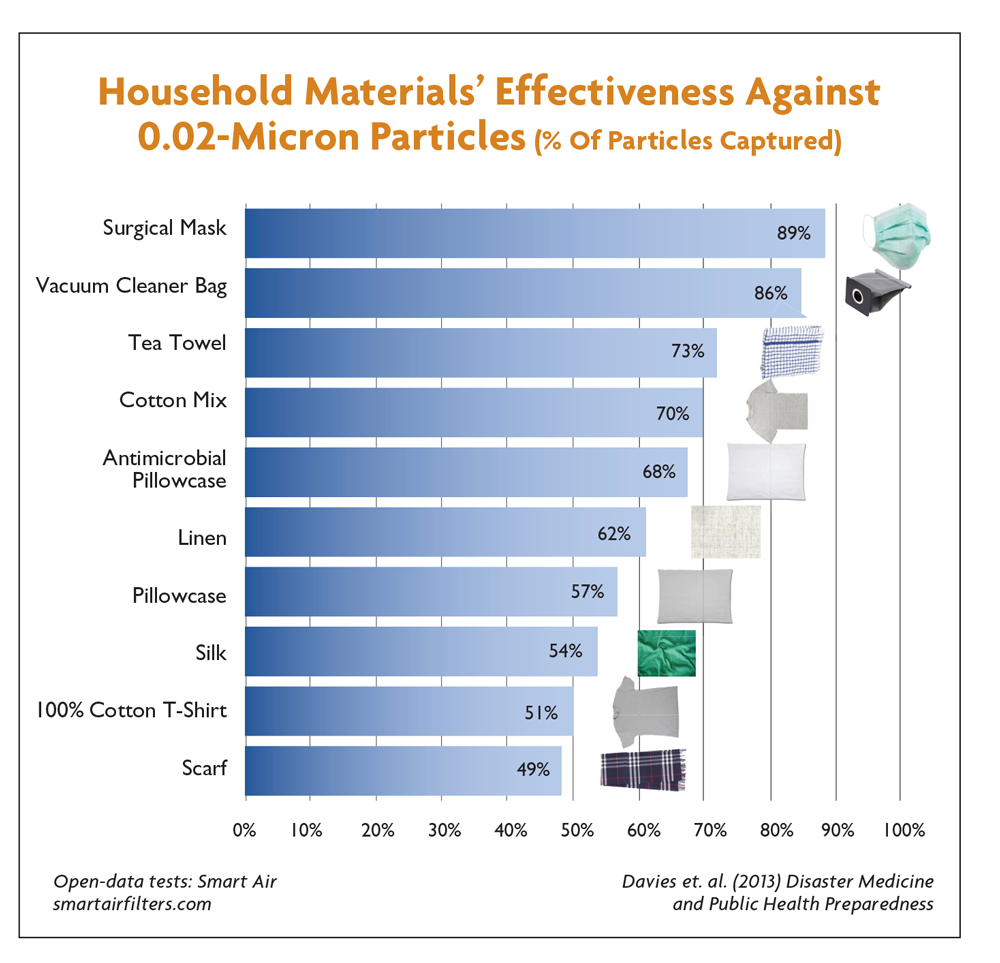
Another study that examined DIY face coverings produced from readily available fabric types provides useful guidance regarding construction characteristics. Davies, Anna, et al, evaluated the filtration efficiency of a variety of household materials, including a cotton T-shirt, scarf, tea towel, and vacuum cleaner bags, against two microorganisms chosen to be representative of the influenza virus and published the results in the paper “Testing the efficacy of homemade masks: would they protect in an influenza pandemic?”26
The study showed the pillowcase and 100-percent cotton T-shirt to be the most suitable household materials for constructing DIY masks. Moreover, the doubling of fabric layers did not significantly increase the filtration efficiency but did negatively affect breathability. The filtration of these DIY face coverings was also compared to surgical masks. While the DIY face coverings were better than controls — no face covering — the improved filtration efficiency from surgical masks was three times that of DIY masks for blocking the transmission of microorganisms via coughing.
Understanding Fabric Characteristics For Protective Face Coverings
Existing literature provides guidance on how fabric characteristics impact protective face covering performance. For example, researchers have shown that DIY cloth face coverings can have varied pore sizes, shapes, and distributions.24 These studies support the general understanding that fabrics with larger pore sizes provide a lower level of filtering efficiency.24
In addition to pore size, the number of available pores can be affected by a number of fabric, yarn and fiber characteristics. In general, fabric modifications that increase the number of pores, while making them smaller should improve filtration performance. For instance, simply by increasing the thread count of a fabric, the spaces between yarns — fabric interstices — will be increased in number while made smaller in size. This should improve filtration efficiency. However, breathability may be negatively impacted as seen in the study above,26 where an effective increase in fabric interstices is achieved by doubling the fabric layers.
Fiber type, size and shape will also affect the number and size of available pores. Smaller fibers and irregularly shaped fibers — cotton or trilobal man-made fibers, for example — will provide added surface area. With any of these modifications, breathability and comfort must also be considered.
Fabric Characteristics May Be Impacted By Re-Wearing, Laundering And Disinfecting
Filtering efficiency of face coverings may be adversely affected by re-wearing and laundering, as well as by repeated fit adjustment. Researchers have found that washing, drying, and/or stretching of cloth face coverings may influence the pore characteristics and subsequent filtering efficiency.24
Because of the current shortage of N95 respirators, researchers have investigated the potential for disinfecting and reuse, despite general guidelines which do not support reuse of such equipment in normal circumstances.28,29 Scientists have identified that certain disinfecting methods such as autoclaving, >70% alcohol, chlorine-based disinfectants, and high temperature dry heat treatments at 165°C, reduce the filtration efficiency of N95 respirators. Instead, disinfection methods such as dry heat at 70°C, boiling water vapor, vaporous hydrogen peroxide, and ultraviolet light treatments appear more promising to maintain function in emergency circumstances associated with equipment shortages.29.30
Opportunity To Define New Standards
Although PPE has entered the common language, the associated regulatory definitions are not well understood. Navigating the regulatory requirements for PPE can a be daunting and time-consuming undertaking. But even with these challenges, there is opportunity for the textile community to participate in defining new standards and specifications for face coverings. PPE and surgical masks have specific performance and regulatory requirements that are overseen by governmental entities or other certifying agencies. On the other hand, DIY face coverings that are currently recommended by the CDC do not have performance requirements and are not regulated. Recent publications provide useful information on the important aspects of fabric construction and filtration performance for face coverings. They show that fiber type, fiber geometry and fabric construction play an important role in performance and comfort, and that there is an opportunity to systematically describe textile characteristics that make this possible.
References:
* SARS-CoV-2 is the name that the World Health Organization has given to the virus that causes the COVID-19 disease. See https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
1 The authors are consultants in the Polymer Science and Health Science practices at Exponent, Inc., a science and engineering consulting firm. This publication was authored by employees of Exponent, Inc. No portion of this publication has been funded or paid for by external sources.
2 For the purposes of this article, “protective coverings” means textiles that cover a portion of the user’s body that may reduce the risk of transmitting or contracting and infection from SARS-CoV-2 or other infectious agents. This includes Personal Protective Equipment (PPE) regulated by governmental agencies in workplaces and general coverings (e.g., face coverings) recommended by other agencies, such as the CDC. PPE use and effectiveness as it is associated with SARS-CoV-2 transmission is still under study in the scientific community.
3 “Recommendation Regarding the Use of Cloth Face Coverings, Especially in Areas of Significant Community-Based Transmission” Centers for Disease Control and Prevention, reviewed 3 April 2020, www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html. Accessed 21 April 2020.
4 https://www.osha.gov/SLTC/personalprotectiveequipment/
5 https://www.fda.gov/about-fda/fda-basics/what-does-fda-regulate
6 https://www.cdc.gov/niosh/about/default.html
7 N95 respirators rely upon complex polymeric fiber arrangement, static charge, and a tight custom fit to the wearer.
8 “Surgical N95 vs. Standard N95 – Which to Consider?” 3M Personal Safety Division Technical Bulletin, Revision 2, March 2020. multimedia.3m.com/mws/media/1794572O/surgical-n95-vs-standard-n95-which-to-consider.pdf. Accessed 29 April 2020.
9 “Key Differences Between Respirators and Masks” 3M Personal Safety Division, April 2014. multimedia.3m.com/mws/media/956213O/differences-between-respirators-and-masks.pdf?fn=Respirator%20vs%20Surgical%20Mask%20flye. Accessed 29 April 2020.
10 “Infection Prevention Solutions – Face Masks and Respirators” 3M Healthcare. multimedia.3m.com/mws/media/312703O/masks-and-respirators-tri-fold-brochure-eng.pdf. Accessed 29 April 2020
11 “Content Details 21 CFR 878.4040 – Surgical apparel” U.S Government Publishing Office, www.govinfo.gov/app/details/CFR-2004-title21-vol8/CFR-2004-title21-vol8-sec878-4040/summary. Accessed 29 April 2020
12 “A Comparison of Surgical Masks, Surgical N95 Respirators, and Industrial N95 Respirators” Occupational Health and Safety, 1 May 2014, ohsonline.com/articles/2014/05/01/comparison-respiratory.aspx. Accessed 20 April 2020
13 Desai, Angela and Mehrotra, Preeti. “Medical Masks.” JAMA Network, 4 March 2020. 10.1001/jama.2020.2331. Accessed 14 April 2020
14 https://www.osha.gov/SLTC/personalprotectiveequipment/standards.html
15 Enforcement Policy for Face Masks and Respirators During the Coronavirus Disease (COVID-19) Public Health Emergency (Revised), April 2020.
16 “Personal Protective Equipment for Infection Control” U.S. Food & Drug Administration, 10 February 2020, www.fda.gov/medical-devices/general-hospital-devices-and-supplies/personal-protective-equipment-infection-control. Accessed 29 April 2020.
17 bin‐Reza, Faisal, et al. “The use of masks and respirators to prevent transmission of influenza: a systematic review of the scientific evidence.” Influenza and other respiratory viruses 6.4 (2012): 257-267.
18 https://www.cdc.gov/niosh/npptl/topics/respirators/pt84abs2.html
19 ANSI/ISEA Z87.1-2015, American National Standard For Occupational And Educational Personal Eye And Face Protection Devices.
20 ANSI/ASSP Z88.2-2015, Practices For Respiratory Protection.
21 ASTM F2100-19, Standard Specification for Performance of Materials Used in Medical Face Masks.
22 Jansen, K. “Why the best material for a homemade coronavirus face mask is hard to identify?” Chemical & Engineering News April 2020. Available at: https://cen.acs.org/biological-chemistry/infectious-disease/best-material-homemade-coronavirus-face/98/web/2020/04
23 Qin, Yimin, ed. Medical textile materials. Woodhead Publishing, 2015.
24 Neupane, Bhanu Bhakta, et al. “Optical microscopic study of surface morphology and filtering efficiency of face masks.” PeerJ 7 (2019): e7142.
25 National Academies of Sciences, Engineering, and Medicine. “Rapid Expert Consultation on the Effectiveness of Fabric Masks for the COVID-19 Pandemic.” The National Academies Press (2020) 10.17226/25776.
26 Davies, Anna, et al. “Testing the efficacy of homemade masks: would they protect in an influenza pandemic?” Disaster medicine and public health preparedness 7.4 (2013): 413-418.
27 Neupane, Bhanu Bhakta, et al. “Optical microscopic study of surface morphology and filtering efficiency of face masks.” PeerJ 7 (2019): e7142.
28 “Enforcement Policy for Sterilizers, Disinfectant Devices, and Air Purifiers During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency. Guidance for Industry and Food and Drug Administration Staff” U.S. Food & Drug Administration, March 2020.
29 “Decontamination and Reuse of Filtering Facepiece Respirators.” Centers for Disease Control and Prevention, reviewed 9 April 2020. www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html. Accessed 29 April 2020
30 Price, Amy and Chu, Larry. “What are good ways to address the shortage of face masks by anesthesiologists?” Stanford Medicine via Learnly (March 2020)
Editor’s Note: Dr. Davis Lee is senior managing scientist, Dr. Erin Kirkpatrick is managing scientist, Dr. A. Sydney Gladman is manager, and Dr. Emily Fitzharris is associate in the Polymer Science & Materials Chemistry practice of Exponent Inc. — a multi-disciplinary engineering and scientific consulting firm. Michael Posson is senior managing scientist and Dr. Mark Roberts is principal scientist at Exponent’s Health Sciences practice.
May/June 2020