 TOKYO — August 4, 2023 — Azides are chemical compounds widely used in synthetic organic chemistry, pharmaceutical sciences, and materials chemistry. However, conventional synthesis methods for azide compounds have severe limitations. To this end, a team of researchers from Japan has developed an efficient method to prepare chemical intermediates having protected “azido” groups. The team successfully synthesized several azides by the Grignard reaction, paving the way for the synthesis of a broad range of organonitrogens (amines and triazoles) for industrial applications.
TOKYO — August 4, 2023 — Azides are chemical compounds widely used in synthetic organic chemistry, pharmaceutical sciences, and materials chemistry. However, conventional synthesis methods for azide compounds have severe limitations. To this end, a team of researchers from Japan has developed an efficient method to prepare chemical intermediates having protected “azido” groups. The team successfully synthesized several azides by the Grignard reaction, paving the way for the synthesis of a broad range of organonitrogens (amines and triazoles) for industrial applications.
Azide compounds play a pivotal role for subsequent synthesis of organonitrogens such as amines and triazoles that are essential compounds in organic and materials chemistry. Triazoles that can be synthesized by the click reaction have attracted attention in the development of pharmaceuticals and other industries. However, the azido groups are electrophilic and are susceptible to various nucleophiles such as carbanions. This poses a significant challenge for the synthesis of carbanions having azido groups.
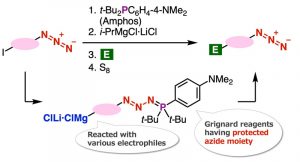
To this end, a team of researchers from Japan, led by Associate Professor Suguru Yoshida from Tokyo University of Science (TUS), has now developed an innovative and efficient azide synthesis method. In their recent article published in the journal Frontiers in Chemistry, Dr. Yoshida and his colleagues Rina Namioka (TUS) and Minori Suzuki (Tokyo Medical and Dental University) have detailed an efficient method to prepare organomagnesium (organic compound containing a magnesium ion linked to a carbon) intermediates having a protected azido group. This paper was published in Volume 11 of the journal Frontiers in Chemistry.
“We conducted this research because we believe that the range of azide compounds that can be easily synthesized can be expanded by developing a synthesis method based on the azide group protection method that we discovered,” Dr. Yoshida said.
The novelty of this synthesis method lies in protection of azido groups with di(tert-butyl)(4-(dimethylamino) phenylphosphine (Amphos) and following iodine-magnesium exchange realized the preparation of organomagnesium intermediates, which served in the synthesis of diverse azides by transformations with various electrophiles followed by deprotection with elemental sulfur. In this study, the authors found a new azide synthesis method utilizing the Grignard reaction and developed a new method for the synthesis of 1,2,3-triazoles.
Specifically, the team found that the iodine-magnesium exchange reaction proceeds efficiently with aryl iodides having azido groups by using the ‘azide group protection method’ that they have recently developed. Speaking about the underlying mechanism that enabled the researchers to achieve this novel azide synthesis method, Dr. Yoshida recalls, “The Grignard reaction of organomagnesium compounds that were synthesized by this reaction succeeded in the synthesis of a wide range of azide compounds.”
As azide compounds play a central role in the synthesis of 1,2,3-triazoles in click chemistry, this method is expected to contribute to the efficient development of pharmaceuticals and other industrially important products.
“Our lab is currently conducting research on the preparation and transformation of carbanions with phosphazide moieties to expand the toolbox of such compounds,” notes an optimistic Dr. Yoshida. The seamless, efficient synthesis of azides facilitating subsequent synthesis of a broad range of organonitrogen compounds such as amines and triazoles will immensely benefit synthetic organic chemistry, pharmaceutical sciences, and materials chemistry communities.
Posted August 8, 2023
Source: Tokyo University of Science




