Cationic Pretreatments Of Cotton
Fiber treatment before dyeing reduces environmental pollutants, increases dye
affinity. Despite the widespread acceptance and demand for cotton apparel, there are
still some significant problems remaining with the wet processing of cotton fabrics.Considerable
amounts of harsh chemicals and hot water are required to properly desize, scour and bleach cotton.
Once the fabrics have been adequately prepared, dyeing processes for cotton suffer from some
serious environmental difficulties. The most convenient dye classes for cellulose direct dyes and
fiber reactive dyes both require significant amounts of electrolytes to achieve even reasonable
exhaustion rates. These water-soluble anionic dye molecules do not have strong enough affinities
for the fiber without the presence of up to 100 grams per liter (g/L) of either sodium chloride or
sodium sulfate in the dye bath. Once exhausted to the fiber, direct dyes have only fair
washfastness with after-treatments. Since fiber reactive dyes will react with water as well as with
the cotton fiber, as much as 50 percent of the dye is hydrolyzed and must be scoured from the
fabric in order to reach acceptable fastness levels.A fiber treatment applied before dyeing that
would leave the cotton fibers with cationic charges would seem to be a possible solution to both of
these problems. D. M Lewis and X. Lei have reviewed this approach1, and recent work has used
cationic pretreatments to achieve union dyeings of cotton/wool blends2,3.Until the mid-1980s, use
of pigments to color textiles had been generally limited to pad applications because of the
inability of pigments to exhaust to fibers. Since that time, a number of researchers have looked at
cationic pretreatments of fabric prior to exhausting pigments4-6, and the process of exhausting
pigments to garments has become important commercially7,8.
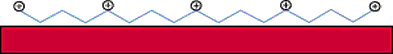
Figure 1. Diagram of process by which cationic polymers forma layer of cationic charges when
applied to fiber surfaces.
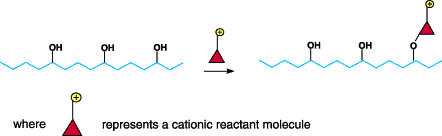
Figure 2. Diagram of process by which reactants modifythe fiber by forming covalent bonds
with the cellulosic fiber. Chemistry Of Cationic PretreatmentsThere are two fundamentally
different types of cationic pre-treatments in use. The first type are cationic polymers that form a
layer of cationic charges when applied to fiber surfaces (See Figure 1).The second category of
cationic pretreatments involves reactants that modify the fiber by forming covalent bonds with the
cellulosic fiber (See Figure 2). The presence of cationic charges on or in the fiber causes anionic
materials such as direct and fiber reactive dyes to be strongly attracted to the fiber and to be
held much more tightly than without the cationic charges.Many types of polymeric cationic
pretreatments have been developed. Some of the chemistries employed include polyacrylates4,
polyimidazoles, polyamideepichlorohydrin resins1, and polyamino condensates2. Their actual
compositions for the most part are proprietary.Reactant cationic pretreatments are usually small
molecules that can form covalent bonds with cellulose. Two examples of chemicals that have been
studied are choline chloride4 and N-(3-chloro-2-hydroxypropyl) trimethyl-ammonium chloride9.
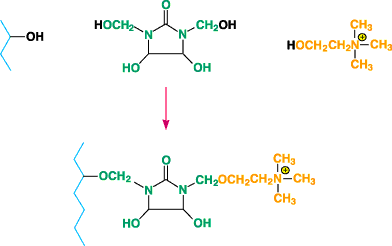
Figure 3. Diagram of modified fiber formed when choline chloride is reactedwith DMDHEU and
catalyst in the presence of cotton.
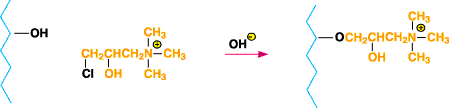
Figure 4. Diagram of cationically modified cotton when
N-(3-chloro-2-hydroxypropyl)trimethylammonium chloride is reacted with cellulose under alkaline
conditions.

Figure 5. Diagram of end result when cationic pretreated cotton is pigment
dyed. Application Of Cationic PretreatmentsCationic pretreatments have been applied to
textiles in two general ways: padding and exhaustion. Polymeric pretreatments have been
incorporated into durable press formulations2,4 applied to fabrics prior to dyeing. Garments can be
treated with cationic polymers with an exhaustion procedure carried out just prior to dyeing5-8.
The efficiency of this exhaustion treatment is directly affected by process parameters such as pH,
temperature, time and agitation level.The goal of exhausting cationic polymers is to provide a
uniform layer of polymer on the fabric surface. Excessive buildup of polymer on the interior
machinery surfaces can occur if the exhaustion process is not carefully controlled. The surface of
the fabric should be clean and free from contaminants such as wax and size in order to assure a
proper pretreatment.Reactant pretreatments have been successfully applied to fabrics by pad
applications3,4,9,10. At present, the most economical reactant pre-treatments are small
water-soluble molecules that do not readily exhaust to cotton fabric. As a result, exhaustion
techniques have not been utilized as extensively as padding procedures. Recent patents, however,
demonstrate continued interest in application of cationic reactants to garments11,12.Cationic
reactants have been attached to cellulose by several mechanisms. For example, choline chloride has
been reacted with DMDHEU and catalyst in the presence of cotton to give a modified fiber according
to the scheme shown in Figure 34.N-(3-chloro-2-hydroxypropyl) trimethylammonium chloride has been
reacted with cellulose under alkaline conditions to yield cationically modified cotton directly10
(See Figure 4).In all cases, uniform application of the cationic reactant to the fabric prior to
chemical reaction with the fiber is essential. Unlevel dyeings are the inevitable results of uneven
applications of cationic pretreatments. Pigment Dyeing Of Cationic Pretreated CottonExhaust
dyeing of cationic pre-treated cotton with pigments is a three-step process:application of
pretreatment (exhaust or pad);exhaustion of pigment; andexhaustion of a binder.Pigments intended
for exhaust application to cationically pretreated fabrics are designed to be anionic or nonionic
when dispersed in water. The exhaustion of these pigments is controlled not only by the pigment
particles charge, but also by the pH and temperature of the dye bath, the length of exhaustion
cycle and the agitation level in the dyeing machine.Polymeric binders are used to increase the
durability (washfastness and crocking) of the pigment-dyed fabric. These binders are usually
cationic in nature and are exhausted to the fabric by controlling treatment bath pH and
temperature.The end result of this process is a multi-layered structure that can be represented by
the diagram shown in Figure 5.The colorfastness of pigment-dyed garments is strongly dependent on
the interactions among pretreatment, pigment and binder. Considerable development work is often
necessary to arrive at a commercially acceptable product.A typical pigment garment-dyeing procedure
is given in Table I. The procedure can vary depending on the machinery used and the specific
pretreatment, pigments and binder chosen. Chemical suppliers will provide the actual
details. Dyeing Of Cationic Pretreated Cotton With Anionic DyesAdding cationic charges
to cotton fiber greatly increases the exhaustion rate and exhaustion level of anionic dyes.
However, this increased affinity can lead to unlevel dyeings unless the dyeing process is carefully
controlled. The uniformity of the dye strike is influenced by the pH and temperature of the dye
bath and the rate of addition of the dyes. Salt is not required for complete exhaustion, and much
higher color yields (approxi-mately 25 percent higher) are seen. Afterscouring of reactive dyes is
usually not necessary, and direct dyes do not require an aftertreatment to achieve acceptable
washfastness. A typical procedure for dyeing cationic pretreated cotton with anionic dyes is
outlined in Table II. SummaryPolymeric cationic pretreatments for cotton offer several
advantages for the garment wet-process industry. Pigments can be exhausted to garments, allowing
styling options not easily obtained by other processes. Existing equipment can be utilized for
these pretreatments because the polymers exhaust to the fabric surface. Disadvantages to be
considered are the fact that the procedure is a complex multi-step process, and often the
lightfastness of anionic dyes is adversely affected by the pretreatment.Reactant cationic
pretreatments for cotton also offer several advantages for the garment wet processor. Fiber
reactive dyes can be used without salt, and the amount of unfixed dyes is greatly reduced.
Significant energy savings can be realized by the reduction of the amount of hot water needed for
the process. Currently, however, the most efficient way of applying reactant cationic
pre-treatments to garments is a fabric application in which pretreated sewing threads are used with
pretreated fabrics during garment manufacture. In addition, the dye procedure must be carefully
controlled to ensure level dyeings.As the reduction of pollutants in textile wastewater grows in
importance, interest in the use of cationic pretreatments for cotton can be expected to grow
also. References1Lewis, D. M. and X. Lei, Textile Chemist and Colorist, Vol. 21, No. 10,
October 1989, 23.2Cardamone, J. M., G. Bao, W. M. Marmer, R. L. Dudley and J. Bulan-Brady, Textile
Chemist and Colorist, Vol. 28, No. 12, December 1996, 19.3Cardamone, J. M., W. M. Marmer, E. J.
Blanchard, A. H. Lambert and J. Bulan-Brady, Textile Chemist and Colorist, Vol. 28, No. 11,
November 1996, 19.4Harper, R. J., Jr., Journal of Coated Fabrics, Vol. 18, No. 4, April 1989,
234.5Angliss, I. and R. Blair, Australasian Textiles, Vol. 10, No. 4, July/August 1990, 56.6Chong,
C. L., S. Q. Li and K. W. Yeung, American Dyestuff Reporter, Vol. 81, No. 5, May 1992, 17.7AATCC
Garment Wet Processing Technical Manual, 1994, 106.8Cotton Dyeing and Finishing: A Technical Guide,
Cotton Incorporated, 1996, 74.9Rupin, M., Textile Chemist and Colorist, Vol. 8, No. 9, September
1976, 54.10Patton, R. T., J. D. Kitchens and D. M. Hall, U. S. Patent 5,006,125 (1991).11Hall, D.
M., T. M. Leonard, C. D. Cofield and H. W. Barrow, U.S. Patent 5,330,541 (1994).12Hauser, P. J. and
S. G. Helfrich, U.S. Patent 5,667,533 (1997).
Editor’s Note: Peter Hauser, Ph.D., is an associate professor of textile chemistry at North
Carolina State University College of Textiles, Raleigh, N.C. His research focuses on
high-performance chemical finishes for enhanced value textiles, indigo dyeing and denim garment wet
processing, new textile processes to reduce costs and energy use and pollution associated with wet
processing. Dr. Hauser holds several patents. He can be reached by telephone at (919) 513-1899 or
by e-mail at peter_hauser@ncsu.edu.The original text of this article was presented at the Emerging
Technologies and Trends in Garment Wet Processing Symposium, New Orleans.
October 2000




